-Visit OBiO Tech booth #1644 at American Society of Gene & Cell Therapy in Maryland, USA
SHANGHAI, April 29, 2024 /PRNewswire/ -- OBiO Technology (Shanghai) Corp., Ltd. (OBiO, 688238.SH), a world leading contract development and manufacturing organization for cell and gene therapy, will showcase at American Society of Gene & Cell Therapy in Maryland, USA from May 7th to 11th. 9 posters will be presented at American Society of Gene & Cell Therapy.
Among these presentations are three self-developed patented achievements, four notable advancements in process optimization, and two cutting-edge technology introductions.
These milestones exemplify OBiO Technology's significant strides in the gene therapy domain, enhancing vector yield and efficiency, refining therapeutic processes, and driving down production costs.
The innovative solutions offered by OBiO Technology provide dependable and cost-effective production methods for gene therapy, propelling forward clinical projects in gene and cell therapy.
OBiO Technology's experts are ready to share how their comprehensive viral vector platform accelerates development timelines, de-risks manufacturing, and provides the performance and product quality needed for your success, with a lower cost.
"We are delighted to continue our legacy of excellence at the ASGCT conference! OBiO Technology will participate with utmost enthusiasm and innovative prowess, showcasing our latest achievements in gene and cell therapy." said Javier Jia Guo, Ph.D., Chief Executive Officer of OBiO Technology. "Our nine presentations, featuring self-developed patented achievements, process optimization breakthroughs, and technology introductions, epitomize our commitment to relentless innovation and collaborative problem-solving. They also underscore our significant progress in enhancing yield efficiency, streamlining production processes, and driving cost efficiencies. OBiO Technology remains steadfast in its forward-looking technological layout and continuous improvement of service capabilities, contributing to the rapid advancement of the gene and cell therapy industry."
About OBiO Technology
OBiO Technology leads the way in gene and cell therapy as a pioneering Contract Research Organization (CRO) and Contract Development and Manufacturing Organization (CDMO), dedicated to providing comprehensive solutions.
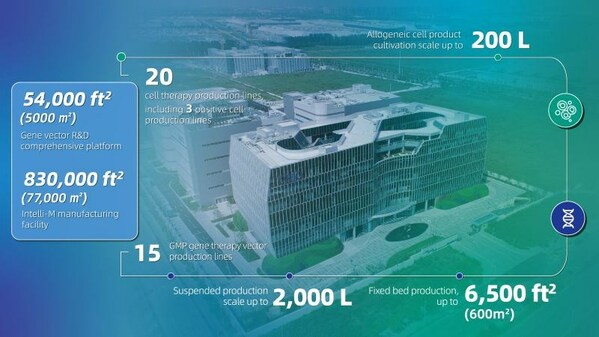
- OBiO Technology's state-of-the-art 830,000 sq.ft facility ensures global supply excellence.
- Specializing in vectorology studies, functional genomics, and process and analytics development, OBiO Technology ensure Investigational New Drug (IND) readiness and support all phases of clinical and commercial manufacturing.
- Guided by mission to "Enable Gene Therapy for Better Lives," OBiO Technology prioritize delivering top-tier services worldwide.
- From laboratory to clinic, OBiO Technology continuously advance your products, making a positive impact on populations worldwide.
- OBiO Technology's unwavering commitment ensures the delivery of high-quality CDMO services across the preclinical, IND, clinical, and commercial stages.
- OBiO Technology's expertise spans plasmids, mRNA, AAV, LVV, Ad viral vectors, cell therapy (CAR-T, NK, Treg), as well as cutting-edge technologies like inducible viral vector packaging, ultralow endotoxin processes, and tissue-specific AAV variants.
Copyright © acrofan All Right Reserved














 [CES 2021] LG Emphasized "Needs to Innovate Beyond..
[CES 2021] LG Emphasized "Needs to Innovate Beyond.. [CES 2021] Sony Unveiled Its Latest Initiatives Sur..
[CES 2021] Sony Unveiled Its Latest Initiatives Sur.. Macan sets new standards: the first all-electric S..
Macan sets new standards: the first all-electric S.. Putting the new Macan through its paces in the nam..
Putting the new Macan through its paces in the nam.. G-Star 2019 : Global Game Exhibition for the Whole..
G-Star 2019 : Global Game Exhibition for the Whole.. 2019 LoL Champions Korea Spring Media Day
2019 LoL Champions Korea Spring Media Day DISNEY+ Showcases Ambitious New Content Slate from..
DISNEY+ Showcases Ambitious New Content Slate from.. eCommerce leader Coupang Dives into Video Streamin..
eCommerce leader Coupang Dives into Video Streamin..